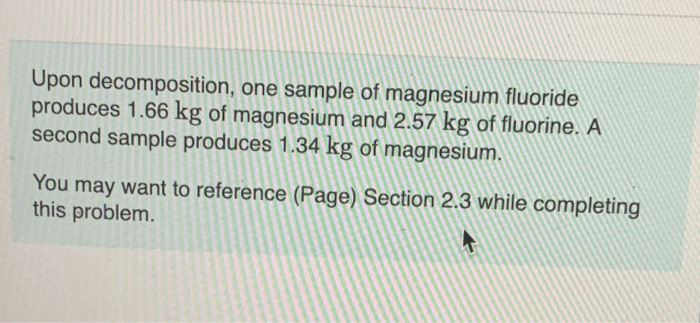
Upon decomposition one sample of magnesium – In the realm of chemical reactions, the decomposition of magnesium presents a captivating spectacle. As we delve into the intricate world of this process, we embark on a journey to unravel the mysteries surrounding this fascinating element. From its chemical properties to its diverse applications, this exploration promises to illuminate the transformative nature of magnesium upon decomposition.
Magnesium, with its inherent reactivity and penchant for forming ionic bonds, undergoes a remarkable transformation when subjected to specific conditions. This process, known as decomposition, yields products that hold valuable insights into the composition and structure of this versatile material.
Chemical Properties of Magnesium
Magnesium is a highly reactive metal with a strong tendency to form ionic bonds. It readily reacts with other elements, including oxygen and water, to form stable compounds. The chemical properties of magnesium are largely determined by its low ionization energy and high electronegativity.
Reactivity of Magnesium
- Magnesium is a highly reactive metal that reacts with most non-metals, including oxygen, chlorine, and sulfur.
- It reacts with acids to form salts and hydrogen gas.
- Magnesium reacts with water to form magnesium hydroxide and hydrogen gas.
Formation of Ionic Bonds
Magnesium forms ionic bonds by transferring two electrons to other atoms or molecules. This results in the formation of positively charged magnesium ions (Mg 2+) and negatively charged anions. The ionic bonds formed by magnesium are typically strong and stable.
Decomposition of Magnesium
Decomposition is a chemical process in which a compound breaks down into simpler substances. Magnesium can decompose under certain conditions, such as when it is heated to a high temperature in the absence of oxygen.
Conditions for Decomposition
- Magnesium decomposes when it is heated to a temperature above its melting point (650 °C).
- The decomposition reaction occurs in the absence of oxygen.
Products of Decomposition
When magnesium decomposes, it breaks down into its constituent elements: magnesium and oxygen. The reaction can be represented by the following equation:
2Mg(s) → 2Mg(g) + O 2(g)
Analysis of Decomposed Magnesium: Upon Decomposition One Sample Of Magnesium
The products of magnesium decomposition can be analyzed using various techniques, such as X-ray diffraction and spectroscopy.
X-ray Diffraction
X-ray diffraction is a technique used to determine the crystal structure of a material. It can be used to identify the phases present in decomposed magnesium and to determine their relative amounts.
Spectroscopy, Upon decomposition one sample of magnesium
Spectroscopy is a technique used to analyze the chemical composition of a material. It can be used to identify the elements present in decomposed magnesium and to determine their concentrations.
Applications of Decomposed Magnesium
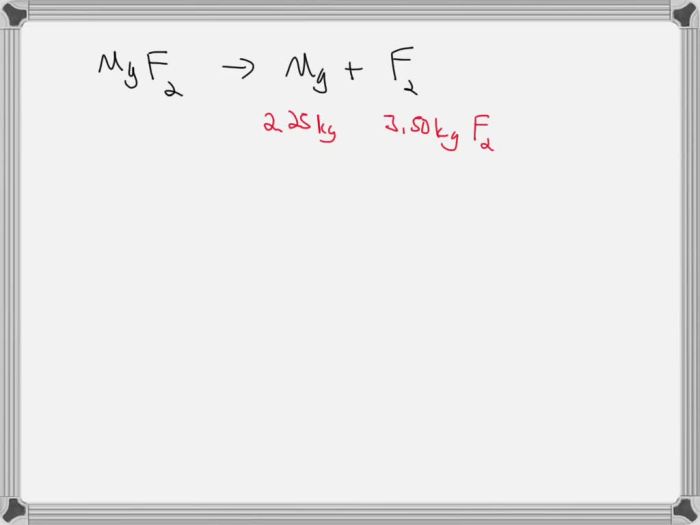
Decomposed magnesium has a number of applications, including:
Refractory Materials
Decomposed magnesium is used as a refractory material in the production of high-temperature furnaces and kilns. It is resistant to heat and corrosion, making it an ideal material for these applications.
Catalyst
Decomposed magnesium is used as a catalyst in a number of chemical reactions. It is particularly effective in the production of hydrogen and in the removal of sulfur from fuels.
Advantages and Disadvantages
- Advantages:
- High melting point
- Resistant to heat and corrosion
- Effective catalyst
- Disadvantages:
- Highly reactive
- Can be expensive to produce
Answers to Common Questions
What factors influence the decomposition of magnesium?
Temperature, pressure, and the presence of other elements or compounds can affect the rate and extent of magnesium decomposition.
How can we identify the products of magnesium decomposition?
Techniques such as X-ray diffraction and spectroscopy provide valuable information about the composition and structure of decomposed magnesium.
What are the practical applications of decomposed magnesium?
Decomposed magnesium finds uses in refractory materials, catalysis, and various industrial processes.