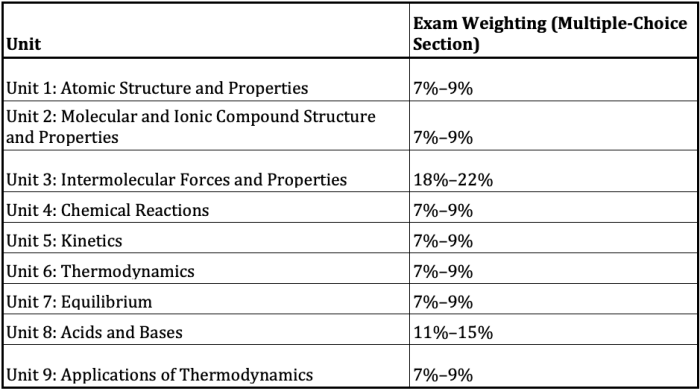
Step into the fascinating world of AP Chemistry Unit 6 FRQ, where we delve into the intricacies of equilibrium, acids and bases, and thermodynamics. This unit offers a comprehensive exploration of chemical principles, providing a solid foundation for your understanding of chemistry.
From Le Chatelier’s principle to the Nernst equation, we’ll uncover the secrets behind chemical reactions and their applications in the real world. Join us on this captivating journey as we unravel the mysteries of chemical equilibrium and beyond.
Equilibrium

Equilibrium is a dynamic state in which the concentrations of reactants and products in a chemical reaction do not change over time. This occurs when the forward and reverse reactions are happening at the same rate. Le Chatelier’s principle describes how equilibrium shifts in response to changes in conditions.
Factors Affecting Equilibrium
Several factors can shift an equilibrium reaction in a specific direction:
- Concentration:Increasing the concentration of reactants shifts the equilibrium to the product side, while increasing the concentration of products shifts the equilibrium to the reactant side.
- Temperature:Increasing the temperature shifts the equilibrium to the side that absorbs heat (endothermic reaction), while decreasing the temperature shifts the equilibrium to the side that releases heat (exothermic reaction).
- Pressure:For reactions involving gases, increasing the pressure shifts the equilibrium to the side with fewer moles of gas, while decreasing the pressure shifts the equilibrium to the side with more moles of gas.
- Addition of a Common Ion:If a common ion is added to a solution, the equilibrium shifts away from the side that contains that ion.
Real-World Application
One real-world application of Le Chatelier’s principle is the Haber process, which is used to produce ammonia for fertilizers. The reaction is:“`N 2(g) + 3H 2(g) <=> 2NH3(g) + heat“`To increase the yield of ammonia, the following conditions are used:
- High pressure:Shifts the equilibrium to the product side (fewer moles of gas).
- Low temperature:Shifts the equilibrium to the exothermic product side.
- Removal of ammonia:As ammonia is produced, it is removed from the reaction mixture, shifting the equilibrium to the product side.
Acid-Base Equilibria
Acids and bases are two fundamental classes of compounds that play crucial roles in many chemical and biological processes. Acid-base equilibria involve the reactions between acids and bases, which can result in the formation of salts and water. Understanding acid-base equilibria is essential for various applications, including analytical chemistry, environmental science, and biochemistry.
pH and pOH
The pH and pOH of a solution are two important parameters that measure the acidity or basicity of the solution. pH is defined as the negative logarithm of the hydrogen ion concentration ([H+]), while pOH is the negative logarithm of the hydroxide ion concentration ([OH-]):
pH =
log[H+]
pOH =
log[OH-]
The pH and pOH of a solution are related by the following equation:
pH + pOH = 14
At 25°C, a neutral solution has a pH of 7, indicating that the concentrations of H+ and OH- ions are equal. A solution with a pH less than 7 is acidic, while a solution with a pH greater than 7 is basic.
Relationship between pH and Acid/Base Strength
The strength of an acid or base can be determined by its pH or pOH. Strong acids have a low pH, indicating a high concentration of H+ ions, while weak acids have a higher pH, indicating a lower concentration of H+ ions.
Similarly, strong bases have a high pH, indicating a high concentration of OH- ions, while weak bases have a lower pH, indicating a lower concentration of OH- ions.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a useful tool for calculating the pH of a solution of a weak acid or base. The equation is:
pH = pKa + log([A-]/[HA])
where:
- pKa is the acid dissociation constant of the weak acid
- [A-] is the concentration of the conjugate base of the weak acid
- [HA] is the concentration of the weak acid
The Henderson-Hasselbalch equation can be used to calculate the pH of a solution of a weak acid or base at any given concentration of the acid or base and its conjugate base.
Solubility Equilibria
Solubility refers to the ability of a substance to dissolve in a solvent to form a homogeneous mixture. The solubility product constant (Ksp) is a quantitative measure of the extent to which a solid substance dissolves in a liquid. It is the equilibrium constant for the dissolution reaction of a solid compound.
The solubility of a compound is affected by several factors, including temperature, pressure, and the nature of the solvent. In general, the solubility of a compound increases with increasing temperature and decreases with increasing pressure. The nature of the solvent also plays a role, with polar solvents tending to dissolve polar compounds and nonpolar solvents tending to dissolve nonpolar compounds.
Applications of Solubility Equilibria
Solubility equilibria have numerous applications in various fields, including chemistry, geology, and environmental science. One important application is in the precipitation of solids from solutions. By controlling the conditions of a solution, such as temperature and pH, it is possible to selectively precipitate desired solids.
This principle is used in various industrial processes, such as the production of pharmaceuticals and pigments.
Thermodynamics

Thermodynamics is the branch of chemistry that deals with the energy changes that occur during chemical reactions and physical processes. It is a fundamental science that has applications in many fields, such as engineering, biology, and environmental science.
Entropy
Entropy is a measure of the disorder or randomness of a system. The more disordered a system is, the higher its entropy. Entropy is always increasing in a closed system, which means that the universe is becoming increasingly disordered over time.
The relationship between entropy and spontaneity is that spontaneous processes are those that increase the entropy of the universe. For example, the reaction of hydrogen and oxygen to form water is spontaneous because it increases the entropy of the universe.
Thermodynamic Processes
There are four main types of thermodynamic processes:
- Isothermal processesare processes that occur at constant temperature.
- Adiabatic processesare processes that occur without any heat transfer between the system and the surroundings.
- Isochoric processesare processes that occur at constant volume.
- Isobaric processesare processes that occur at constant pressure.
Gibbs Free Energy Equation, Ap chemistry unit 6 frq
The Gibbs free energy equation is a mathematical equation that can be used to predict the spontaneity of a chemical reaction. The equation is:
ΔG = ΔH
TΔS
where:
- ΔG is the change in Gibbs free energy
- ΔH is the change in enthalpy
- T is the temperature in Kelvin
- ΔS is the change in entropy
If ΔG is negative, the reaction is spontaneous. If ΔG is positive, the reaction is nonspontaneous.
The AP Chemistry Unit 6 FRQ is a challenging exam, but it’s also an opportunity to show off your knowledge of the subject. One way to prepare for the exam is to practice writing out the names of chemical compounds.
This can help you improve your spelling and your understanding of the periodic table. If you’re looking for a fun way to practice, try finding words with p o i s e m . There are many different words that contain these letters, so you’re sure to find some that you can use in your chemistry studies.
Electrochemistry
Electrochemistry delves into the fascinating world of chemical reactions that involve the transfer of electrons. This field plays a crucial role in our daily lives, from powering our electronic devices to the processes that occur in our bodies.
Oxidation and Reduction
Oxidation and reduction are two inseparable processes that occur simultaneously. Oxidation refers to the loss of electrons, while reduction signifies the gain of electrons. These processes are central to the chemical reactions that take place in electrochemical cells.
Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa. There are two main types of electrochemical cells: galvanic cells and electrolytic cells.
- Galvanic cellsproduce electrical energy from spontaneous chemical reactions. A classic example is the voltaic pile, where zinc and copper electrodes immersed in an electrolyte generate an electric current.
- Electrolytic cellsuse electrical energy to drive non-spontaneous chemical reactions. A familiar example is electrolysis, where an electric current is passed through a solution to decompose compounds.
Nernst Equation
The Nernst equation is a powerful tool that allows us to calculate the cell potential of an electrochemical cell under non-standard conditions. This equation relates the cell potential to the concentrations of reactants and products, temperature, and the standard cell potential.
E = E°
(RT/nF) ln Q
where:
- E is the cell potential under non-standard conditions
- E° is the standard cell potential
- R is the ideal gas constant
- T is the temperature in Kelvin
- n is the number of electrons transferred in the balanced chemical equation
- F is the Faraday constant
- Q is the reaction quotient
Detailed FAQs: Ap Chemistry Unit 6 Frq
What is Le Chatelier’s principle?
Le Chatelier’s principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that counteracts the change.
What is the pH of a solution?
pH is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity.
What is the solubility product constant (Ksp)?
The solubility product constant is a constant that expresses the equilibrium concentration of the ions of a sparingly soluble salt in a saturated solution.